Sources of Light
The visible spectrum of light is just a small part of the electromagnetic spectrum which extends from radio waves (long waves, kilometers in extent) to gamma rays (10-3 meters down to 10-15 meters, the size of the nucleus). The more familiar x-rays have wavelengths around 10-10 meter. The visible spectrum lies in the middle range with wavelengths between 400 nanometers (nm) for blue and 700 nm for red. The receptors of the eye respond to light in the visible spectrum and, as shown in Figure 1, the sun's spectrum encompasses the visible spectrum. Light from the sun is absorbed by the earth's atmosphere in the ultraviolet (UV) and infrared (IR) but some reaches the earth. The UV portion that causes sunburn can be blocked by glass and much of the light is blocked by water except for the blue (B) and violet portions.
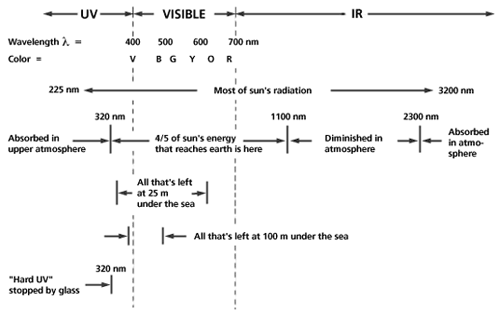
Figure 1: The region of the sun's spectrum that spans the range from ultraviolet (UV) to infrared (IR). Different portions of the sun's energy are absorbed in the atmosphere, water, and glass.
Human daylight vision is most effective in the blue-green (around 550 nm) where the sun's energy spectrum is in the region of its maximum. Figure 2 shows the spectrum of the sun along with those of a tungsten lamp and a candle flame, both incandescent light sources where incandescence refers to light produced by the temperature of an object.
In a candle, the wax is melted by the heat of the flame, flows up the wick and is vaporized. Combustion raises the temperature of the carbon particles to incandescence and causes the emission of the yellow color. As shown in Fig. 2 the maximum in the visible spectrum is at the long wavelength, red, end.
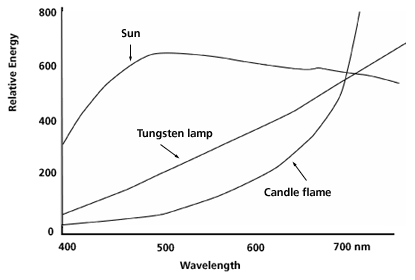
Figure 2: Energy spectrum in the visible region for the sun, tungsten lamp, and candle flame.
In the tungsten lamp, the electric current runs through the filament which becomes hot and radiates. As with the candle some of the radiation is in the visible but most is in the infrared (IR). To keep the tungsten filament from burning or melting, the glass bulb is filled with a mixture of argon and nitrogen gases that does not react with tungsten.
Fluorescent lamps operate by a different principle from incandescence, a gas discharge. A glass tube is filled with mercury vapor and others, such as sodium, and the electrodes are connected to an alternating current (AC) source. The electric source ionizes the atoms in the tube which emit light primarily in the UV. The inside of the tube is coated with phosphors which absorb UV light and produce visible light. Consequently, fluorescent tubes have an energy spectrum with a broad spectrum, similar to the tungsten lamp, and a series of sharp intense peaks in the red, blue, and green. The intense green line (mercury vapor) at 546 nm is used to calibrate spectrometers.
Want to try activities highlighting these principles? Click on the name of this activity to find an easy, hands-on experiment:









